Industries
Military — Robust and reliable HMI components for extreme requirements

HMI components in military use must withstand the highest loads and function reliably in extreme environments. At EP Electronic Print, we develop customized solutions that are suitable for military applications. Our products offer robustness, precision and reliability to meet the requirements of land, air and naval forces.
EMC protection is also guaranteed for displays and touch sensors to prevent interference in sensitive environments. We offer the production of complex assemblies in small to medium quantities, flexibly and precisely tailored to your requirements. We also handle the procurement of discontinued components and compatible replicas in accordance with the fit-form-function (3F) criteria to ensure long-term functionality and integration.
Areas of application in the military
Our HMI components can be used in numerous military applications, including:
Special requirements and features
Medical technology places specific requirements on the components used to ensure optimum functionality and patient safety. Our solutions meet these requirements through:
Closed surfaces
Medical devices have special requirements in terms of reliability and the protection of users and patients. In order to avoid malfunctions due to the ingress of solids or liquids, medical devices are normally designed with closed surfaces or, in the case of medical devices, at least the IP-Schutzklasse 65 gem. ISO EN 60529 recommended.

Resistance to disinfectants and cleaning agents
Frequent cleaning and disinfection is a permanent challenge for the materials used. The materials we use have proven themselves in practice over many years and therefore represent a safe basis(DIN 42115). Sometimes, however, applications require special cleaning agents (e.g. highly alkaline or acidic). We will be happy to support you with additional data on various resistances or suggest a suitable solution.

Optimized readability
Depending on the application, the readability or accuracy of the representation on the display can be a very sensitive issue. The choice of the right components, the right optical specification and the type of connection (e.g. optical bonding or air-gap bonding) must be selected with a view to the target. Neither “overshooting the target” nor “just doing it” are optimal in terms of price, quality and performance.
Traceability of the functional components
The demands on quality management are constantly increasing and have therefore led to increased safety for patients and users. If problems do occur despite precautionary and risk management measures, complete traceability of the functional components is required in order to analyze problems accurately. We can provide you with the necessary data at serial number level.
Protection against liquids
Thorough cleaning and disinfection of devices is more important in the medical sector and therefore takes place more frequently than in many other applications. Systems in medical technology should therefore be completely resistant to the penetration of cleaning agents and disinfectants, drugs, steam or water. For medical devices, at least the IP-Schutzklasse 65 gem. ISO EN 60529 recommended.

Electromagnetic compatibility — EMC
To ensure patient safety, IEC 60601–1‑2 specifies increased requirements for the EMC behavior of medical devices. This affects factors such as interference and radiation, conducted coupling, etc. The importance of the right concept with the selection of mechanical and electronic hardware cannot be emphasized enough. In the worst-case scenario, there is a risk of reworking the final device.

Usability with gloves
The use of gloves is common practice in all medical areas for reasons of hygiene. The latex disposable gloves frequently used in medical technology are no problem for any of our input systems and can be used without any problems. However, thicker gloves made of various materials such as cotton or nylon can also be considered if required.

Mechanical resistance of the surface
Modern multi-touch HMI systems are usually equipped with a stable glass surface and are therefore already very robust against mechanical impact from the outside. But even if glass cannot or should not be used (e.g. for tactile feedback), we only use films with hardened surfaces (3H or 9H according to ISO 15184) and thus maximize the mechanical resilience.

Anti-microbial surfaces
Microbes, especially bacteria, are a persistent problem in applications with high hygiene requirements. We can therefore offer the option of an anti-microbial coating for our products. This reduces the microbial load by 97% within 24 hours(ISO 22196). Although this does not replace regular, professional cleaning, it drastically reduces the risk of microbial spread.

Manufacturing
ISO 13485 is a harmonized standard that formulates the requirements for quality management (QM) and the QM system of medical device manufacturers. We are happy to supply medical device manufacturers who manufacture in accordance with this standard and also provide you with all the necessary documents and information.
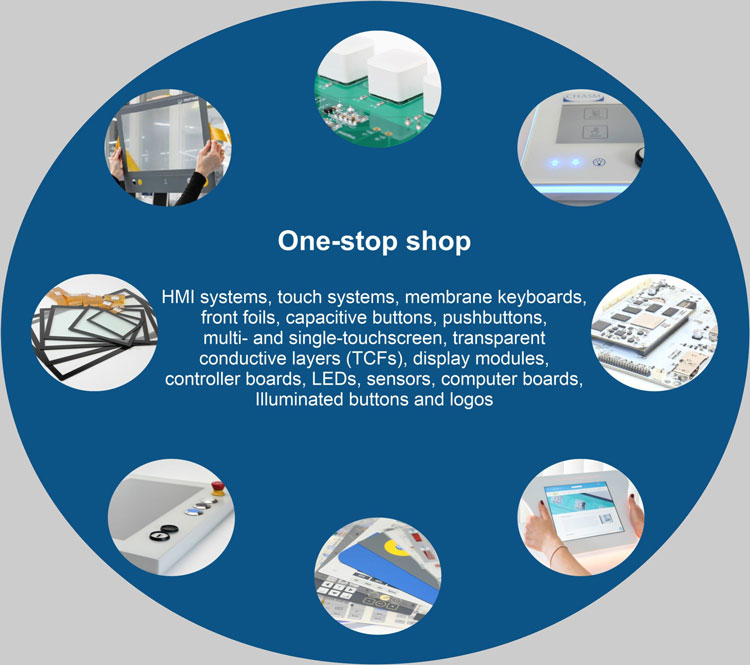
Why EP Electronic Print?
At EP Electronic Print, we rely on state-of-the-art components and technologies to develop innovative and customized HMI solutions.
Do you have any questions or would you like to find out more about our HMI solutions?
Our team will be happy to advise you and work with you to find the best solution for your project. Use our contact form or call us directly — we look forward to hearing from you!
Non-binding contact request
Am Weidegrund 8 & 10
82194 Gröbenzell Technische Anfragen:
Telefon: +49 (0)8142 /420896–20
E‑Mail: projekte@ep-electronicprint.de Allgemeine Anfragen:
Telefon: +49 (0)8142 /420896–0
E‑Mail: info@ep-electronicprint.de

